Mass: the amount of matter in a given area- NOT the same as weight (labeled as grams, kg, mg, etc.)
Volume: the amount of space an object takes up (labeled as mL, kL, L, etc. or cm cubed, m cubed, etc.)
How to find density of a solid, regular shaped object: Put the object on a balance and record the mass of it in grams. Then measure the length, width and height of the object with a ruler in centimeters.
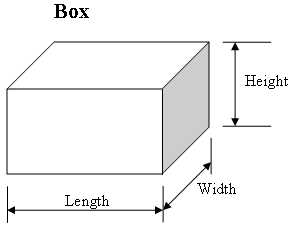
Multiply all of these numbers together. For example, let's say the length is 5 cm, the width is 2 cm, and the height is 4 cm. So then you would multiply 5 cm x 2 cm x 4 cm, which would equal 40 cm cubed. Next, take the mass and divide it by the volume. For example, if the mass was 45 g, it would be 45g divided by 40 cm cubed. Your final answer will be 1.125 g/cm cubed.
How to find density of an irregular shaped object: Put the object on a balance and record the mass of it in grams. Since you aren't able to measure the length, width, or height of an irregular shaped object, you will have to use a method called water displacement. To do this, fill up a graduated cylinder big enough to fit your object in. Record what measurement is on the graduated cylinder in mL. Make sure you read the measurement at eye-level from the meniscus. A meniscus is the curve of liquid in a graduated cylinder. Read the meniscus from the lowest point, as shown below.
Once you have recorded the "before" measurement, place the object in the water. Then record the new measurement, reading at eye-level from the meniscus. Now you have your "before" measurement, which we will say is 43mL, and your "after" measurement which we will say is 54mL. Subtract the second measurement from the first measurement. So you would subtract 54mL-43mL, which equals 11mL. This will be your volume. Next, take the mass and divide it by the volume. For example, if the mass was 10g, it would be 10g divided by 11mL. Your final answer is .91 g/mL.
How to find density of a liquid: To find the mass of a liquid, put an empty graduated cylinder on the balance. Zero out the balance by pressing the "Zero" button, so the mass of the graduated cylinder is not added in for the liquid's mass. The reading should now say 0 g. Now pour your liquid into the graduated cylinder. Record the measurement in g. Next, find the volume of the liquid by reading the graduated cylinder at eye-level from the meniscus. If we use the example picture above, the volume would be 43mL. To find the density, we take the mass divided by the volume. Let's say the mass is 45g, and the volume is 43mL. So 45g divided by 43mL is 1.05 g/mL. This is your final answer.
How to find volume of a person: In my Advanced Science 9 class, we were trying to find the volume of a person. Since a person is an irregular object, we decided that water displacement would be the best method to use. We needed something big enough to fit a person inside, so we used a baby pool with trash can inside. The baby pool was used to catch all the water that overflowed out the top of the trash can (see the picture below).
The first step to finding the volume of a person is to fill the trash can with water all the way to the very top. Once it's full, have the person you are testing step inside the trash can. In our class, the guinea pig was Bree.
A lot of water should spill out of the top of the trash can and into the pool. Scoop up the water from the pool with cups or any thing similar to that, and pour it into 1,000 mL graduated cylinders. Once you fill one graduated cylinder to 1,000 mL with water, record it on a piece of paper by using tally marks. Keep repeating this process and adding more tally marks until all of the water from the pool is gone. Count up the tally marks, and multiply that number by 1,000. (You may not fill the last graduated cylinder with exactly 1,000 mL, so just make a note of it on the side and add that amount in once you have multiplied all the tally marks.) Label your answer in mL, and you're done!
If you would like to see this experiment being done, here is a video of our class dunking Bree into the trash can:
Results: The final measurement for the volume of Bree was 63,005cm cubed. Again, this answer probably isn't 100% accurate because of the errors, but it's close. Another class period did this same experiment with a different girl named Sarah, and their results were 54,520cm cubed. Their measurement was probably a lot more accurate than ours because they didn't have the big error of the hole in the trash can.
How to find density of candy bars: To find the density of a candy bar, you need to repeat the same process as listed above in the How to find density of a solid, regular shaped object section. Measure the length, width, and height of the candy bar in centimeters. You have to keep the wrapper on when doing this part, so try to flatten out the wrapper on the part you are measuring. Once you have gotten the volume of the candy bar, put it on a balance. Record the mass in grams. Then take the mass divided by volume. For example, if your candy bar had a volume of 15 cm cubed, and a mass of 18 g, then the density of the candy bar would be 1.2 g/cm cubed. Then, you are going to repeat these steps 3 different times, using a different candy bar of the same brand each time. For example, if you chose to do a 3 Musketeer, you would find the density of it, go back and trade it in for a new 3 Musketeer, and do these steps again. Once you have found the density of the same candy bar 3 different times, find the average of the 3 densities. To do this, you add up all of the densities and divide it by 3. For example, if one density was 1.2 g/cm cubed, another was 1.3 g/cm cubed, and the third was 1.1 g/cm cubed, then your final answer for the density of the candy bar you chose would be 1.2 g/cm cubed. We do this "finding 3 different densities and averaging process" because you never know if one candy bar is smashed, has a more dense peanut in it (if the candy bar contains peanuts), etc. It is also used to be more precise and accurate, because on one of the density trials you may have made a mistake or made a math error.
Sink or Float? To find out if something sinks or floats, you use density. Water has a density of 1.0. So, if your object's density is less that 1.0, then your object will float in water. If your object's density is greater than 1.0, then it will sink in water. I chose to measure a Fun Size Three Musketeer and a Fun Size Milky Way. According to my information, the Fun Size Three Musketeers would float in water because its density is less than 1.0g/cm cubed. In class, we tested to see if our calculations were correct. We got a beaker full of water and dropped the candy bar inside. The Three Musketeers floated in the beaker, so my calculations were correct.
Next, we checked the Fun Size Milky Way. According to my information, the Milky was would float because its density was less than 1.0g/cm cubed. We put the Milky Way in another beaker, and it sunk, so my calculations were incorrect.
Errors: Some things that may have caused my Milky Way measurement to be incorrect may have been the wrapper. It gets in the way when measuring the length, width, and height of the candy bar, so some measurements may have been incorrect. The added mass of the wrapper and the air between the wrapper and the actual candy may have added to the overall mass, which could have also caused that measurement to be incorrect. There could have also been a math error when trying to multiply, divide, etc.Particle Diagrams: A way to support your hypothesis of sinking or floating is to make a particle diagram. A particle diagram that we used shows you the amount of particles in the water compared to the amount of particles in the candy bar. For example, take a look at the picture below.
This is a particle diagram of a Fun Size Three Musketeers. As you can see, the candy bar (represented by the small blue box made out of tape) is floating on the water line (represented by the blue horizontal line). The particles (represented by the tennis balls) show that there are less particles in the candy bar than in the water. The particles in the water are packed closer together, making the water more dense than the Three Musketeer. If the water is more dense than the Three Musketeer, then the candy will float.
Above is a particle diagram of a Fun Size Milky Way. Since the Milky Way sunk, then the candy bar is sitting at the bottom of the "water beaker." In this particle diagram, the water particles are spread further apart. As you can see, there are more particles packed closer together in the candy bar, making it more dense than the water. If the candy bar is more dense than the water, then it sinks.
How to Stack Liquids: This may sound weird... Stacking Liquids? But it is possible. Just take a look at the picture below.
In science class, we did an experiment to figure out how to stack liquids. We needed to figure out the order in which to put the colored liquids so they did not to mix. In order to successfully stack them, you have to use only one independent variable at a time. Only put two colors in at the same time to see if they mix. If they mix, then do not stack one right on top of each other. If they didn't mix, then you know that you have a pair that can be stacked on top of each other. Many students in our class started randomly throwing colors together, so they had more than one independent variable. Then they could never figure out the correct order. Only using one independent variable at a time and keeping a control color is key to successfully completing this experiment.
Results: The correct way of stacking these liquids is: at the very bottom pour in red (salt water with red food coloring). Next use a pipette and drop in blue (cold water with blue food coloring). After blue is green (hot water with green food coloring). At the very top should be purple (rubbing alcohol with purple food coloring). If you use a pipette and carefully squeeze the liquids in the graduated cylinder, you should be able to successfully stack four liquids!
Miscible and Immiscible: Being miscible means that the substance has the ability to mix or dissolve. Salt and water is an example of being miscible. If you have a cup of water, and you pour in some salt, the salt will dissolve into the water. On the other hand, immiscible means that the substance does not have the ability to mix together. Water and vegetable oil is an example of being immiscible. The water and vegetable oil will never mix together. Even if you shake them, they will eventually spread back out and become separate again. Watch this video below to see how water and vegetable oil do not mix.







No comments:
Post a Comment